
If using this second equation, do NOT switch any of the signs as you are using the reduction potential already. The magnitude of the potential must remain the same. Turning the reaction around doesnt change the relative strengths of the oxidizing or reducing agents. Reduction happens at the cathode (tin(ll) E reduction=0.14) and oxidation happens at the anode (chromium E reduction = -0.91)īefore using this first equation, change the reduction into oxidation by switching the sign (remember the value given is for a reduction half reaction). The identity of the cathode and anode can be remembered by recognizing that positive ions, or cations, flow toward the cathode. To calculate standard cell potential there are two formulas, but both will give you the exact same answer. Net cell reaction: Sn 2+ (aq) + Cr (s) -> Sn (s) + Cr 2+ (aq) The oxidation of iodide ion /- by permanganate ion MnOF in basic solution to yield molecular iodine /2 and manganese (IV) oxide Mn02- b. Electrical current is carried by electrons in the wire and electrodes, but it is carried.

The positive anode attracts anions toward it, while the negative cathode attracts cations toward it. If they don't then you need to multiply by a coefficient. anode (oxidation) or cathode (reduction) is written as a reduction Ex. Balance the following reactions in both acidic and basic media, identify the oxidation and reduction reactions, and identify the oxidizing and reducing agents. The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). To write your net, make sure that electrons 'cancel' on both sides of the equation (they do in this case). You should have determined that the SOA is Sn 2+ and the SRA is Cr. 7- table of selected standard electrode potentials) determine which is your SOA (start on the top left hand side, determine whether you see Cr, Cr 2+, Sn 2+ or Sn first) and which is your SRA (start on the bottom right hand side, what do you see first).
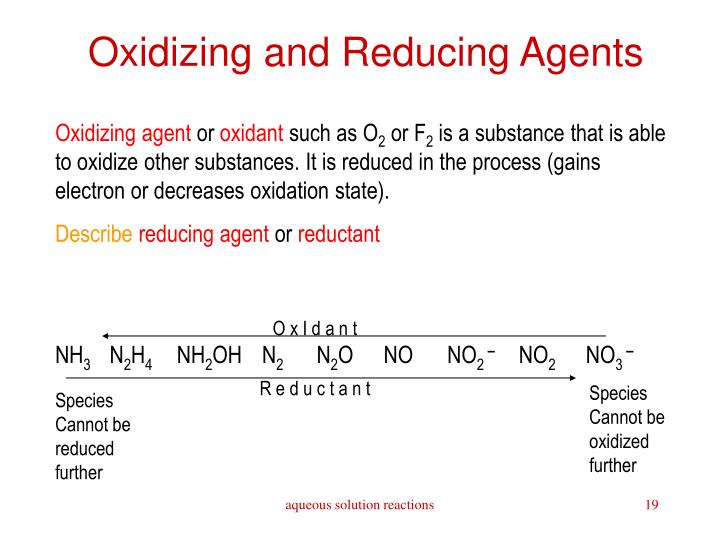
For electrochemical cells, remember that the strongest reducing agent (SRA) is oxidized at the anode (SRAOA) and the strongest oxidizing agent (SOA) is reduced at the cathode (SOARC).


 0 kommentar(er)
0 kommentar(er)
